41 does sugar melt ice
Why Pickle Brine Is a Secret Weapon Against Ice - National Geographic Why Pickle Brine Is a Secret Weapon Against Ice. In the effort to melt ice and snow, salt and calcium chloride have strange—and eco-friendly—company. What Makes Ice Melt Fastest? | Science Project You will test substances that dissolve in water (i.e., soluble substances), like salt and sugar, as well as a substance that does not dissolve in water (i.e., an insoluble substance), specifically sand. Which substances will speed up the melting of the ice? Terms and Concepts Freezing point Phases of matter Freezing point depression Solution Solute
Does sugar or salt melt faster? - populersorular.com If no air is moving around the ice , it cannot warm with the atmosphere and melt faster. 25-Does ice melt faster in milk or water? Ice will melt more quickly in water because water is less dense than either milk or Hershey's Syrup. ( Milk is about 3% more dense than regular water .) 22

Does sugar melt ice
Why Does Sugar Melt Ice? - Reference.com Sugar is able to melt ice because it disrupts the equilibrium of the water molecules and causes the freezing process to slow down, resulting in conditions that favor the melting process over the freezing process. The addition of a foreign molecule, such as sugar, results in a lower freezing point. 7 Ways to Melt Ice Without Using Salt or Ice Melt - Bob Vila A surprising substance with ice-melting capabilities is sugar beet juice. The chemical makeup of beet juice lowers the melting point of ice and snow in a manner similar to rock salt. Not... How does sugar melt ice? - Answers Sugar is just like salt. it increases the melting point so it will melt ice. but the sugar doesn't melt the ice as fast.
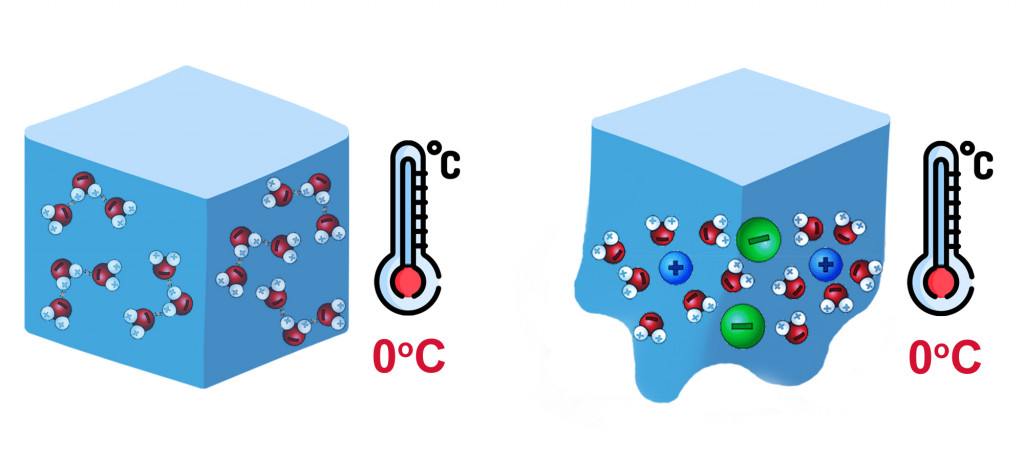
Does sugar melt ice. How does sugar melt ice? - Sage-Answers Salt and sugar make ice melt equally as fast as each other. Sugar, alcohol, salt varieties and other foreign substances all help ice melt faster because they lower the freezing point of the ice. What substances melt ice? The de-icing agents lower the freezing point of water. Science of Cooking: Ask the Inquisitive Cooks! - Exploratorium Sugar lowers the freezing point of water, which makes frozen desserts fair game for changes in freezing point. Most desserts freeze between 29.5 to 26.6 degrees ... Educator Guide: Melting Ice Experiment | NASA/JPL Edu Fill one dish with room temperature water. Measure and record the temperature. Gently place an ice cube in the dish and record how long it takes for the ice cube to melt. There should be enough water in the dish so the ice cube floats. Measure and record the water temperature after the ice has melted. Why Does Sugar Melt Ice Slower Than Salt And How? When dissolved in water, sugar may melt ice, although not as effectively as salt. Sugar often melts ice by reducing water's melting and freezing points, much like salt does—sugar interacts with water molecules by being dissolved on ice. For ice crystals to freeze, water molecules bond to them.
About - What Makes Ice Melt Fastest? Sugar and anything else capable of being dissolved in water will melt ice. Sugar melts ice by lowering water's melting and freezing points, just like salt. 5 Ways to Melt Ice Without Salt | American Home Shield - AHS Sugar is a great homemade de-icer. It de-ices the same way as salt by lowering the freezing point of the water. However, sugar may be more costly than a rock salt ice melt, so you may only want to use it on smaller areas, like your front porch or your back step. 3. Homemade deicer spray for your vehicle's windshield Salt on Ice - Scientific American When ice is surrounded by air or liquid, at room temperature it absorbs heat from its surroundings. As a result the tiny particles in the ice start to vibrate more. Those at the edge might... Why Does Sugar Affect the Freezing Point of Water? | Sciencing Sugar molecules don't pack together with water molecules, so when the water molecules start to freeze, the sugar molecules remain in the liquid water. When the water molecules create ice, the sugar molecules have a smaller volume of liquid in which to move. Freezing Point Depression
Chemistry Explains How Beet Juice Makes Ice and Snow Melt - Inverse The sugar molecules from the beet juice have a similar effect, which means that if beet sugar is added to the 20 percent salt solution and sprayed on ice, the melting point of the ice will be even ... Why Does Sugar Melt Ice Slower Than Salt? - Blurtit I did the experiment for my science fair and I found out that an ounce of ice with sugar on it melts in 2:30 min. While salt melted ice in 1:15 min. How does sugar melt ice? What property makes this happen? - Quora Oct 14, 2016 ... Sugar, like salt, melts ice by reducing the melting and freezing points of water. In order for ice crystals to freeze, water molecules are required to link them ... Melting Ice With Sugar - YouTube Jan 22, 2016 ... I put sugar on the porch to see how good it is at melting ice and snow. Thanks to my patrons this may be the last video I ever make using ...
Why Does Salt Melt Ice? Understanding How It Works - ThoughtCo Salt melts ice and help prevent re-freezing by lowering the freezing point of water. This phenomenon is called freezing point depression. The working temperature range isn't the same for all types of salt. For example, calcium chloride lowers the freezing point more than sodium chloride. In addition to melting ice, freezing point depression can ...
Does salt and sugar affect the melting rate of ice? Sugar is also soluble in water, and also lowered the freezing/melting point of the water, but sugar does not make ice melt as fast as salt does. Flour does not cause the ice cube to melt faster because the flour has almost the same freezing/melting point as pure water.
Why Does Salt Melt Ice? The Interesting Science The simple answer is a term called freezing point depression. Simply put, salt lowers the freezing point of water. When salt dissolves into liquid water, water molecules have a tougher time sticking together and forming ice crystals. On the average driveway, rock salt will typically decrease the freezing point of water to around 15 degrees ...
Melting Ice in Beverages | Physics Van | UIUC One that we've discussed before is that ice melting on salt water or sugar water tends to sit in a pool of very cold recently melted water on top. In water that that doesn't have solutes making it denser, the melted ice mixes more with the rest of the water, speeding the flow of heat to the ice. Mike W. Leh (published on 10/22/2007)
What melts ice the fastest?: Science Course with Storm Team 5 Salt, baking soda, and sugar will all act to lower the freezing point of the ice, making it melt quicker than the untouched ice cube. Sand is another common substance that may be seen on...
How long does sugar take to melt ice? - Answers Does sugar help melt ice? Yes, sugar helps melt ice. It helps melt ice because sugar increases the melting point of the ice which causes it to melt.
Why Does Salt Melt Ice Faster Than Sugar? SCIENCE EXPLAINED Nov 2, 2022 ... Sugar is effective at melting ice down to around 28ºF/-2ºC whereas regular table salt is effective down to about 15ºF/-9ºC and other ice melt ...
How does sugar melt ice? - YouTube May 9, 2022 ... Lesson 17In the final lesson in our Hot & Cold unit, Cheryl learns how making ice cream, deicing roads, and antifreeze are connected.
What Makes Ice Melt Fastest? - Scientific American For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water's freezing point. Both of these events demonstrate "freezing...
12 Drinks To Help Lose Belly Fat, Says Dietitian - Eat This Not That A can of regular soda has about 150 calories and 49 grams of sugar (about 10 teaspoons), while a 12-ounce can of OLIPOP has 2-5 grams of sugar (less than 1 teaspoon), 9 grams of fiber, and 35-45 calories. ... While the beverage won't truly "melt" away fat, there is published research that shows that subjects given acetic acid, the main acid in ...
What Makes Ice Melt Faster? | Science project | Education.com Figure out the average time it takes for ice to melt in plain water, water with salt added and water with sugar added. For further evaluation, try using different amounts of salt. Feel free to experiment with other substances as well, like rubbing alcohol or sand.
Why Does Sugar Melt Ice? | Sciencing If you throw sugar on ice at 30 degrees Fahrenheit (-1.1 degrees Celsius), the ice will melt, but if the temperature falls lower, the water will eventually freeze. The new freezing point is lower than that of pure water, but higher than it would be if you threw salt on the ice. Cite this Article Did you find this page helpful? 👍 👎 References
Why Does Salt Melt Ice? | Britannica When the ionic compound salt is added to the equation, it lowers the freezing point of the water, which means the ice on the ground can't freeze that layer of water at 32 °F anymore. The water, however, can still melt the ice at that temperature, which results in less ice on the roads.
How Salt Melts Ice and Prevents Water From Freezing - ThoughtCo Key Takeaways: How Salt Melts Ice. Salt melts ice and helps keep water from re-freezing by lowering the freezing point of water. This phenomenon is called freezing point depression. Salt only helps if there is a little bit of liquid water available. The salt has to dissolve into its ions in order to work.
How does sugar melt ice? - Answers Sugar is just like salt. it increases the melting point so it will melt ice. but the sugar doesn't melt the ice as fast.
7 Ways to Melt Ice Without Using Salt or Ice Melt - Bob Vila A surprising substance with ice-melting capabilities is sugar beet juice. The chemical makeup of beet juice lowers the melting point of ice and snow in a manner similar to rock salt. Not...
Why Does Sugar Melt Ice? - Reference.com Sugar is able to melt ice because it disrupts the equilibrium of the water molecules and causes the freezing process to slow down, resulting in conditions that favor the melting process over the freezing process. The addition of a foreign molecule, such as sugar, results in a lower freezing point.


:max_bytes(150000):strip_icc()/blocks-of-melting-ice-200025919-001-586a81f93df78ce2c33aa8f9.jpg)









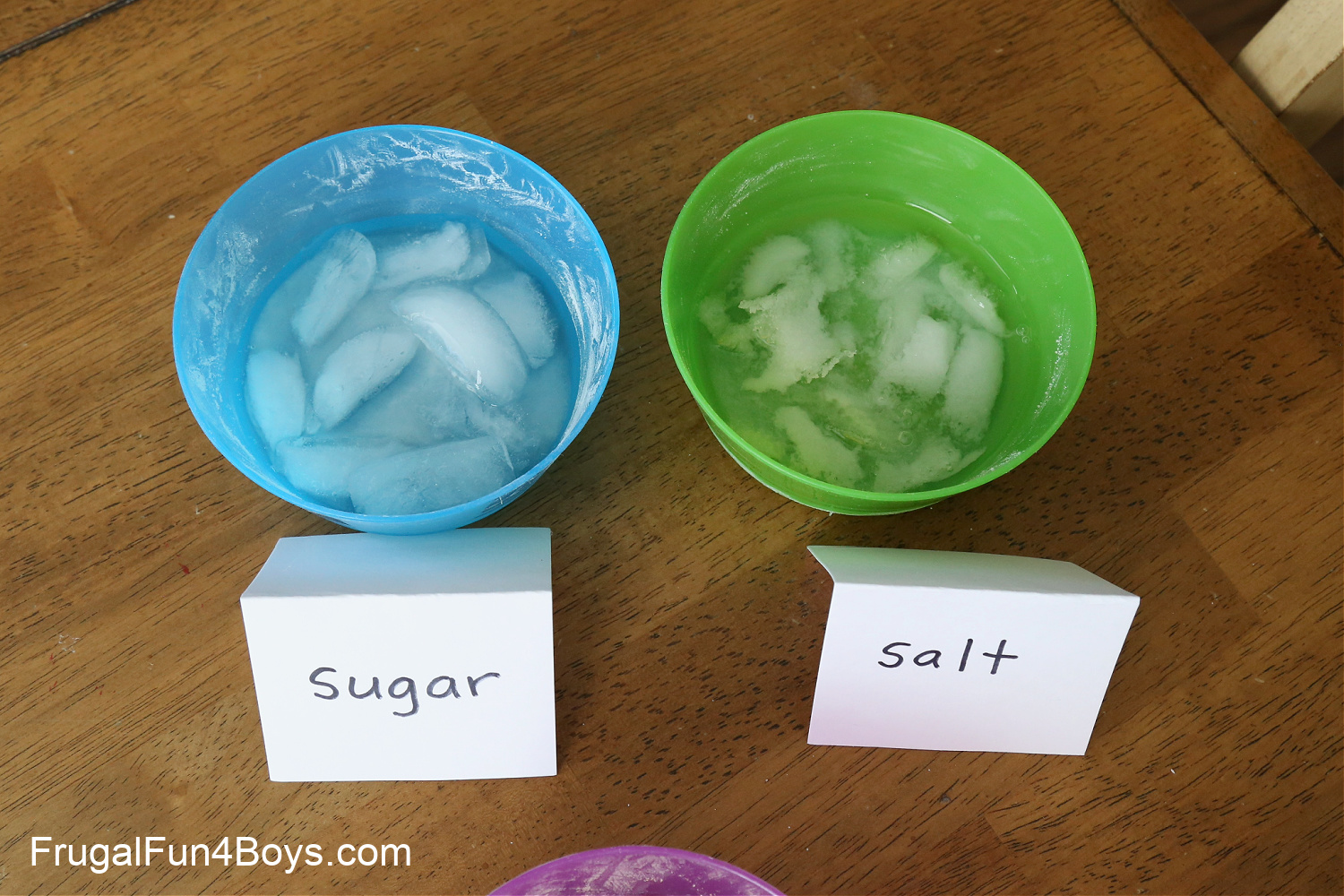









:max_bytes(150000):strip_icc()/two-glasses-full-of-crushed-ice-with-frost-on-outside-of-one-melting-ice-below-and-heap-of-salt-98358220-58a4c7953df78c345b909525.jpg)













0 Response to "41 does sugar melt ice"
Post a Comment