40 how does salt affect water
Water molecules and their interaction with salt | U.S ... - USGS At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges on opposite sides in the molecule. Salt and Sodium | The Nutrition Source | Harvard T.H. Chan School of ... When sodium accumulates in the blood, water is transferred out of cells and into the blood to dilute it. This fluid shift and a build-up of fluid in the brain can cause seizures, coma, or even death. Extra fluid collecting in the lungs can cause difficulty breathing.
With a pinch of salt - Climate Change: Vital Signs of the Planet First, along with temperature, they directly affect seawater density (salty water is denser than freshwater) and therefore the circulation of ocean currents from the tropics to the poles. These currents control how heat is carried within the oceans and ultimately regulate the world's climate.
How does salt affect water
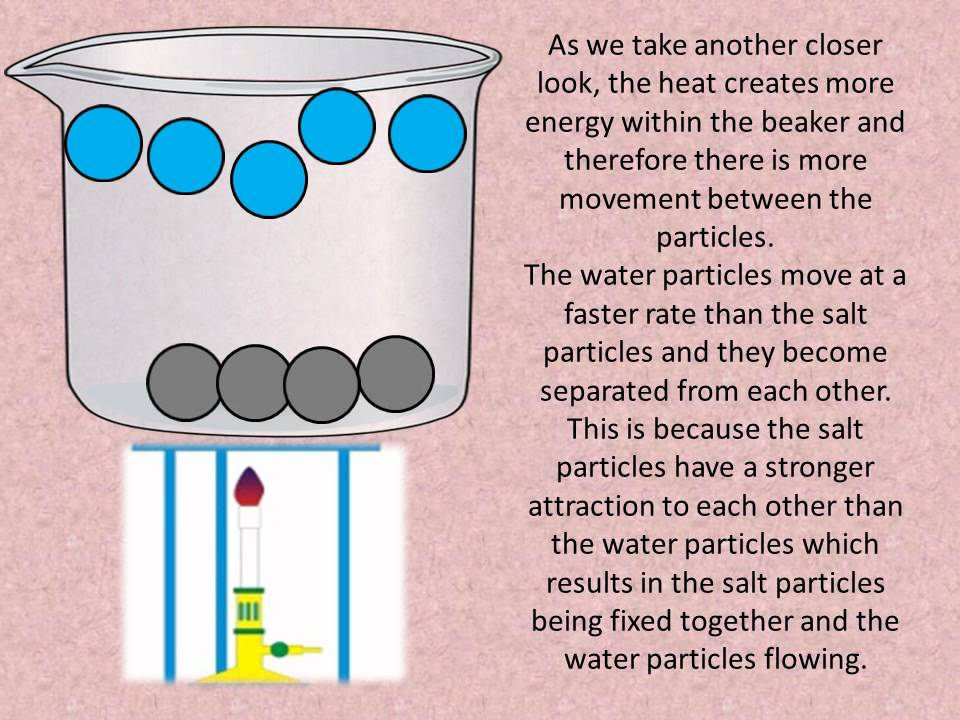
How does salt change the specific heat capacity of water? Increasing the concentration of the salt decreases the specific heat capacity of the water. > When we heat a sample of water, the energy goes into raising the energy levels of its various vibrational, rotational, and translational motions. When we dissolve "NaCl" in water, the ions are held in a rigid cage of water molecules. The cage is rigid enough so that the motions of its molecules are ... How Salt Melts Ice and Prevents Water From Freezing - ThoughtCo Salt melts ice and helps keep water from re-freezing by lowering the freezing point of water. This phenomenon is called freezing point depression. Salt only helps if there is a little bit of liquid water available. The salt has to dissolve into its ions in order to work. Different types of salt are used as de-icing agents. How Does Salt Soften Water? | Rayne Water - Rayne Water conditioning Final Thoughts. Salt is critical for water softening systems that use ion-exchange. These systems remove the minerals in hard water and replace them with sodium ions. This process is gentle, natural, and is excellent for providing soft water to an entire home or building.
How does salt affect water. Water salinity and plant irrigation | Agriculture and Food Salts in irrigation water are mainly common salt (sodium chloride), calcium and magnesium bicarbonates, chlorides and sulphates. In most areas of Western Australia, about three-quarters of the total soluble salt is sodium chloride, though this may vary in coastal and pastoral areas. For example, in irrigation water at Carnarvon, only about half ... Signs You're Eating Too Much Salt - Cleveland Clinic Sodium attracts water. If you eat a lot of salty foods, you'll experience fluid retention (when sodium holds water in your body). The result? You feel swollen and look puffier, especially around the abdomen and eyes. You may also notice swelling in your hands and feet. Increased thirst What Happens When Salt Is Added to Water? | Sciencing When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. At the same time, the slightly electronegative chlorine portion of NaCl is attracted to the slightly electropositive hydrogen portion of water. 8 Effects Living Near Salt Water Has on Your Home & Body Here are a few things to know about the effects of saltwater and air on your body. 1. Skin Living near the sea is great for your skin. Scientists have found that magnesium in ocean water hydrates skin and increases its elasticity.
Why Adding Salt to Water Increases the Boiling Point - ThoughtCo If you add salt to water, you raise the water's boiling point, or the temperature at which it will boil. The temperature needed to boil will increase about 0.5 C for every 58 grams of dissolved salt per kilogram of water. This is an example of boiling point elevation, and it is not exclusive to water. How does salt effect buoyancy? - Reimagining Education When salt is dissolved in water, as it is in ocean water, that dissolved salt adds to the mass of the water and makes the water denser than it would be without salt. Therefore due to increase in density of fluid, upward Buoyant force will increase due to which object will float better in salt water. How much more buoyant is salt water? Does salt have any impact on the aquatic life? | ResearchGate Salt concentration in water or the salinity is very important in aquatic life. The amount of salts (sodium, potassium, carbonates, sulfates) in water and amount of salts in creature's body should ... Chemistry 20IB Internal Assessment.pdf - Research Question: How does ... 2: Background Information The difference between boiling saltwater and boiling pure water is actually quite complicated. This is because, as the quantity of salt added increases the faster the saltwater will heat up; however, the more salt added the higher the boiling point gets (Gegga, 2016). This creates an inverse relationship in which adding salt may increase the rate of heating up but it ...
How Does Salt Affect Heart Health? - Cleveland Clinic Too much salt can cause fluid to build up around the heart and lungs, making the heart work harder. Evidence suggests that a limit of 2,000 mg per day of sodium is a good goal for people with heart failure, especially if they also have high blood pressure. But there's an important caveat. Does Salt Water Evaporate? - Techiescientist On the addition of salt like NaCl in the water, the vapour pressure of the water lowers down. It means that fewer water molecules can escape out of the solution from its surface. Therefore, the higher the concentration of salt present, the lesser is the evaporation. Salt's Effect on Water's Density | Physics Van | UIUC So when we say that salt water is more dense than regular water it means that there is more mass in a certain volume of the salt water than there is in the same volume of normal water. When you add table salt (sodium chloride, NaCl) to water, the salt dissolves into ions, Na + and Cl-. The volume increases by a small factor, but the mass ... Does salt affect the properties of water? [Facts!] A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid as the anion (A−), the conjugate acid of a weak base as the cation (BH+), or both. Salts that contain small, highly charged metal ions produce acidic solutions in water.
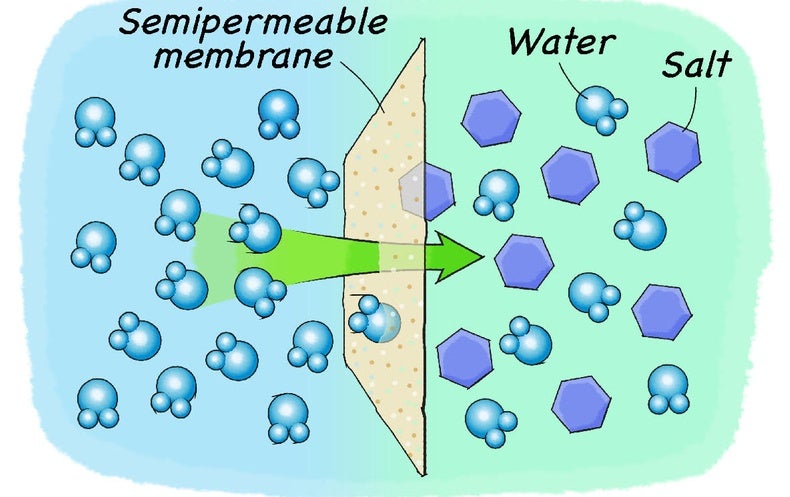
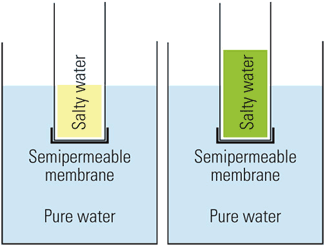
How does salt water affect freshwater? [Facts!] When an ionic salt like NaCl is added to water, the ions from the salt introduced will attract the water molecules in an effort to "solvate" the ions. This has the tendency to decrease the weak affinity of non-polar oxygen molecules to water and drive the dissolved oxygen out of the polar water. What happens when you add salt to water experiment?
Salinity | Science Mission Directorate - NASA As a liquid, water dissolves rocks and sediments and reacts with emissions from volcanoes and hydrothermal vents. This creates a complex solution of mineral salts in our ocean basins. Conversely, in other states such as vapor and ice, water and salt are incompatible: water vapor and ice are essentially salt free.
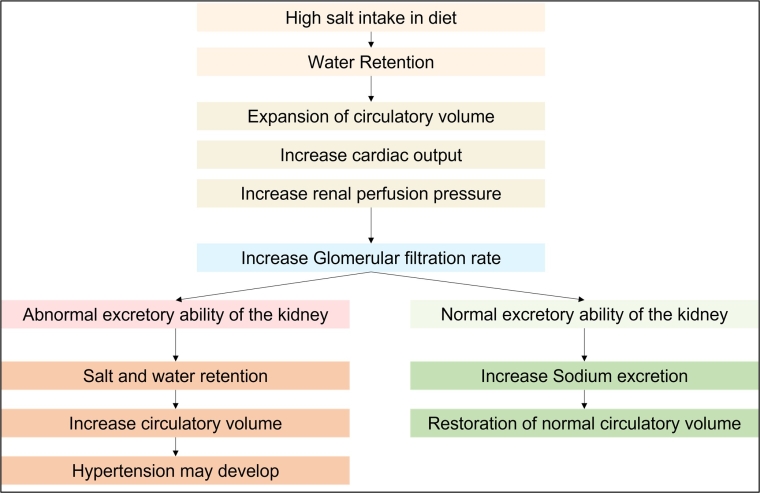
Water Retention: Carbohydrates vs. Salt | livestrong Sodium helps regulate the levels of water in your body. As a result, the total amount of sodium, or salt, you consume has an impact on whether you retain or eliminate water. As you eat more salt, your body holds onto more water, causing that all-too-familiar bloat. Carbohydrates also cause water retention, but in a different way.
What Happens If You Eat Too Much Salt? - Healthline Short-term consumption of high amounts of salt can cause water retention, a temporary rise in blood pressure, excess thirst, and, in severe cases, hypernatremia. However, some people may...
How Does Salt Affect the pH of Water? | Sciencing When it is added to water, it breaks down into ions of sodium and chlorine. Neither of them reacts with water, so salt will only change the volume of the water, not its pH. In order for any type of salt to affect the pH (potential of hydrogen), it has to react with water to release or bind the hydrogen atoms from the water. Acidic Salts
Does salt make water boil faster? | Live Science When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, Giddings said. This gives salt water a...
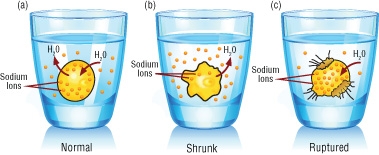
How the body regulates salt levels - National Institutes of Health (NIH) Increasing salt intake increased sodium excretion, but also unexpectedly caused the kidney to conserve water. Excess sodium was thus released in concentrated urine. This method of protecting the body's water was so efficient that the men actually drank less when their salt intake was highest.
Density, Temperature, and Salinity | manoa.hawaii.edu ... The more salt there is dissolved in the water, the greater its salinity. When comparing two samples of water with the same volume, the water sample with higher salinity will have greater mass, and it will therefore be more dense. Temperature Affects Density The density of water can also be affected by temperature.
How Does Salt Affect Ice? A Simple Science Experiment Follow these step-by-step instructions to visualize the effect salt has on the ice. Set the bowls on a level surface. Place an ice cube in each bowl. Label the first bowl as your control. This bowl will only contain an ice cube. Label the second bowl as your variable. Into the second bowl, pour one teaspoon of table salt on top of the ice cube.
How Does Salt Soften Water? | Rayne Water - Rayne Water conditioning Final Thoughts. Salt is critical for water softening systems that use ion-exchange. These systems remove the minerals in hard water and replace them with sodium ions. This process is gentle, natural, and is excellent for providing soft water to an entire home or building.
How Salt Melts Ice and Prevents Water From Freezing - ThoughtCo Salt melts ice and helps keep water from re-freezing by lowering the freezing point of water. This phenomenon is called freezing point depression. Salt only helps if there is a little bit of liquid water available. The salt has to dissolve into its ions in order to work. Different types of salt are used as de-icing agents.
How does salt change the specific heat capacity of water? Increasing the concentration of the salt decreases the specific heat capacity of the water. > When we heat a sample of water, the energy goes into raising the energy levels of its various vibrational, rotational, and translational motions. When we dissolve "NaCl" in water, the ions are held in a rigid cage of water molecules. The cage is rigid enough so that the motions of its molecules are ...



















:max_bytes(150000):strip_icc()/water-in-steel-pan-with-herbs-and-salt-being-added-making-brine-145063802-57a770ea3df78cf459166075.jpg)









0 Response to "40 how does salt affect water"
Post a Comment