41 decomposition of hydrogen peroxide equation
28. Determination of the Rate of the Decomposition of ... Determination of the Rate of the Decomposition of Hydrogen Peroxide* Driving Question. How does changing the concentration of the reactants affect the value of k, the rate law constant? Background. Hydrogen peroxide (H2O2) in aqueous solution decomposes very slowly under ordinary conditions. The equation for the decomposition is (1) A catalyst such as potassium … › reactions › inorganicNa2O2 + H2O = NaOH + H2O2 | Sodium Peroxide and Water Reaction So use algebra equation to find oxidation number of oxygen in Na 2 O 2. Summation of oxidation numbers of all atoms = 0 (+1)*2 + x*2 = 0. x = -1. hydrogen peroxide reaction with sodium hydroxide? hydrogen peroxide does not react with sodium hydroxide. But sodium hydroxide increases the decomposition rate of hydrogen peroxide to water and oxygen ...
The Decomposition of Hydrogen Peroxide | The Chemistry Blog When its oxygen-oxygen bond breaks, hydrogen peroxide decomposes into water and oxygen. When this happens, it releases free radicals that are highly reactive with other substances. While this decomposition reaction can be sped up by a catalyst, the instability of the peroxide bond means that decomposition also occurs naturally.
Decomposition of hydrogen peroxide equation
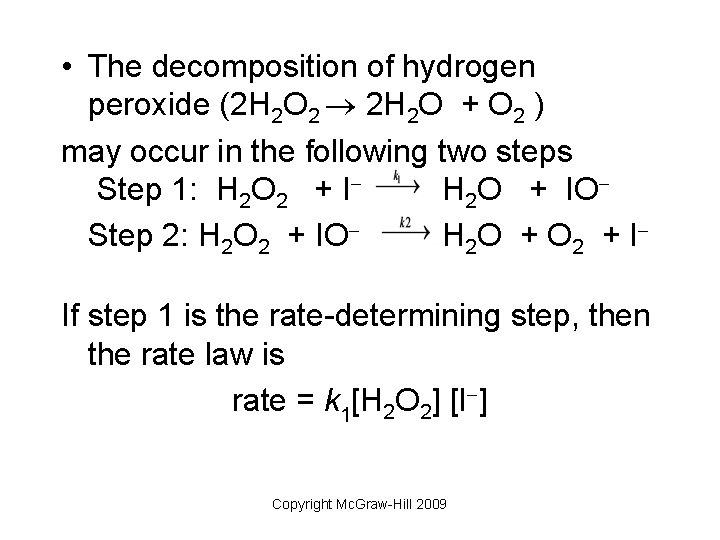
EXPERIMENT 7 Decomposition Kinetics of Hydrogen | Chegg.com This experiment studies the decomposition kinetics of the iodide-catalyzed decomposition of hydrogen peroxide (H2O2) which is thought to proceed through the following mechanisms: 1st step: H2O2 (aq) + (aq) 10 (aq) + H2O (0) 2nd step: H2O2 (aq) + 10 (aq) + (aq) + H2O (l) + O2 (g) Overall reaction equation: 2 H2O2 (aq) + O2 (g) + 2 H2O (1) The ... Is the decomposition of hydrogen peroxide second-order ... Decomposition of Hydrogen Peroxide The decomposition of hydrogen peroxide in aqueous solution proceeds very slowly. A bottle of 3% hydrogen peroxide sitting on a grocery store shelf is stable for a long period of time. The decomposition takes place according to the reaction below. 2 H2O2 (aq) 2 H2O O2 (g) PDF An equation for the decomposition of hydrogen peroxide is ... An equation for the decomposition of hydrogen peroxide is 2H2O2 € 2H2O€€€€+€€€€O2 (a) €€€€The rate of reaction can be determined by collecting the oxygen formed and measuring its volume at regular intervals. Draw a diagram to show the apparatus that you would use to collect and measure the volume of the oxygen formed
Decomposition of hydrogen peroxide equation. Rate equation for the decomposition of hydrogen peroxide ... Rate equation for the decomposition of hydrogen peroxide with iron(III) chloride as catalyst [duplicate] Ask Question Asked 26 days ago. ... I am trying to determine the activation energy of the decomposition of hydrogen peroxide with iron(III) chloride added as a catalyst. To do that, I need to find the rate constant, but I am not sure what to ... The Decomposition of Hydrogen Peroxide | The Chemistry Blog When its oxygen-oxygen bond breaks, hydrogen peroxide decomposes into water and oxygen. When this happens, it releases free radicals that are highly reactive with other substances. While this decomposition reaction can be sped up by a catalyst, the instability of the peroxide bond means that decomposition also occurs naturally. ChemTeam: Synthesis Hydrogen peroxide is made in other ways, NOT by direct union of the elements. Nonetheless, it is a valid synthesis reaction and useful in contexts otherthan how H 2 O 2 is made. Since synthesis reactions are the reverse of decomposition, you might ask if the decomp. categories apply, just in reverse. The answer is yes! Hydrogen peroxide | H2O2 - PubChem Hydrogen peroxide | H2O2 | CID 784 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. COVID-19 Information. Public health information (CDC) Research information (NIH) SARS-CoV-2 data (NCBI) Prevention and ...
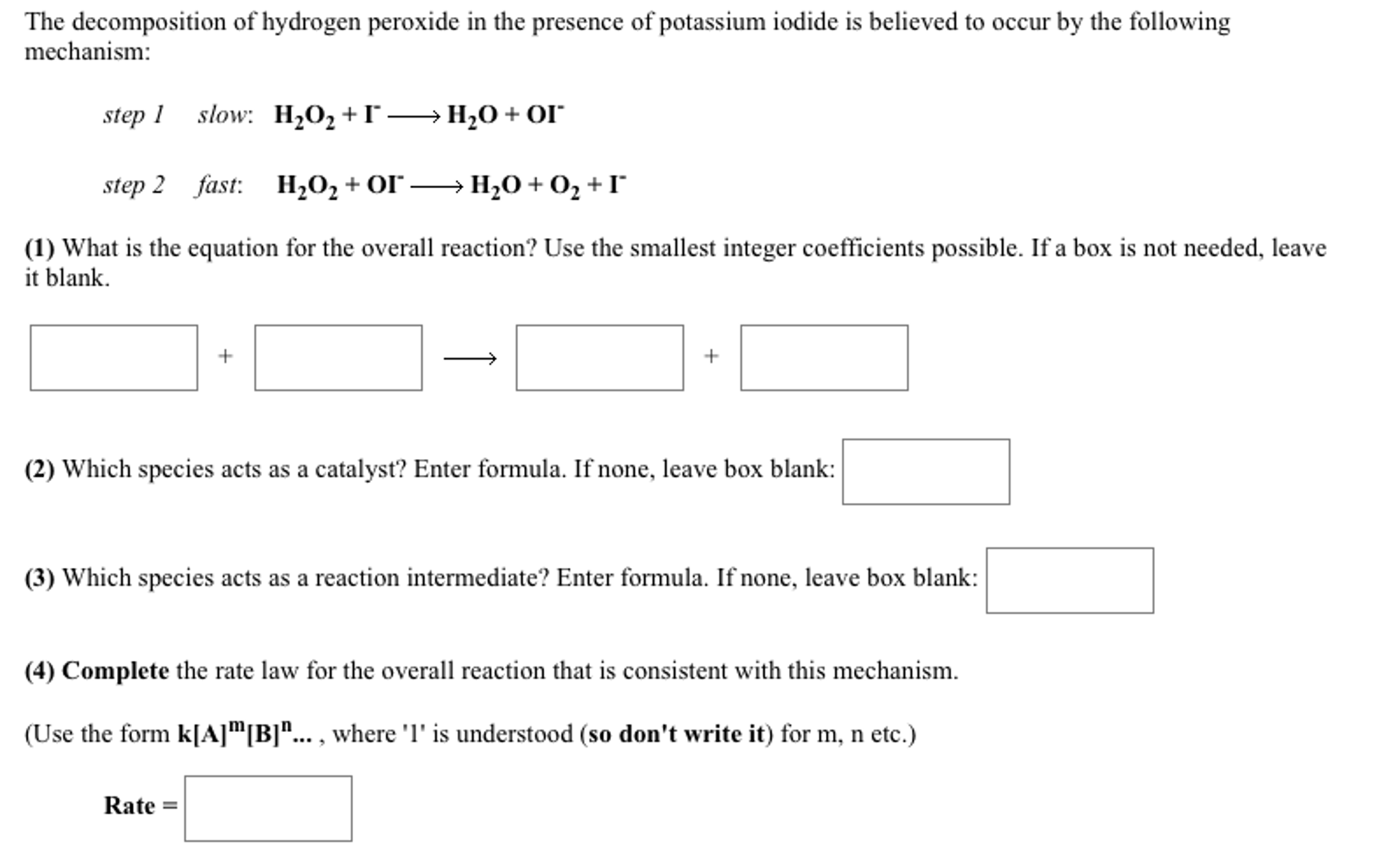
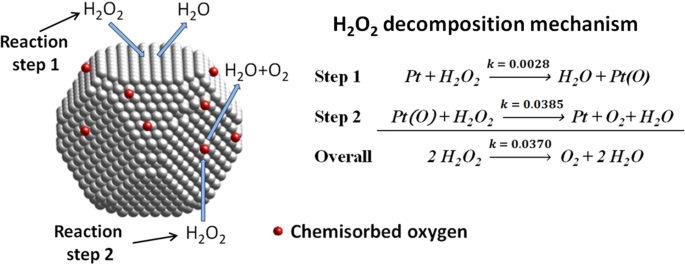
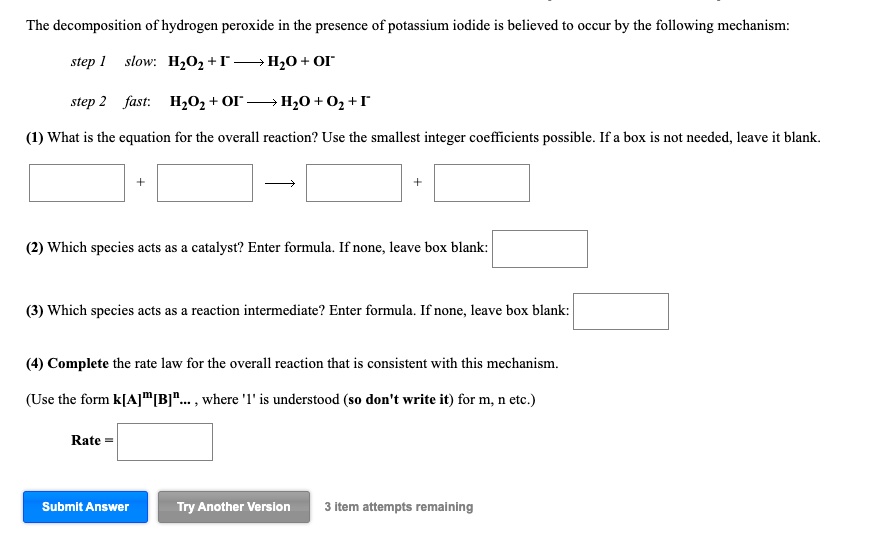
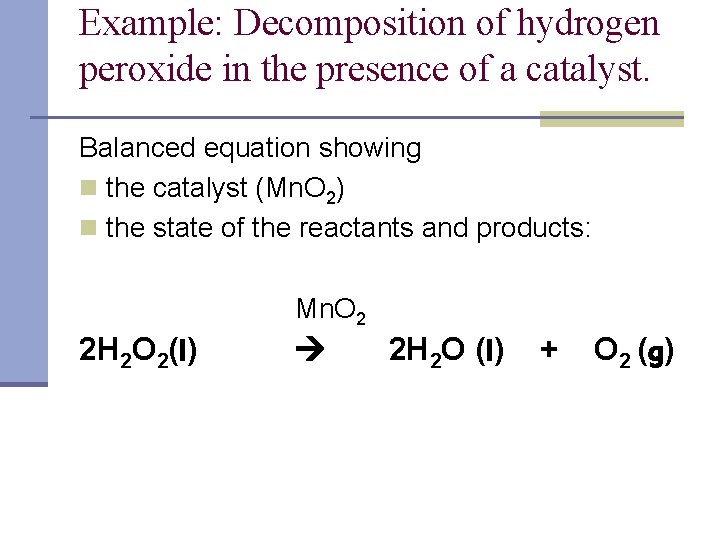
How does iodine affect the decomposition of hydrogen peroxide? When hydrogen peroxide is added to a solution of potassium iodide, the iodide ions are slowly oxidized according to the equation: 22 2 2 potassium iodide hydrochloric acid hydrogen peroxide iodine colorless colorless coloroless yellow-+ iodide Molecular equation: 2KI(aq) + 2HCl(aq) + H O (aq) I (s) + 2H O(l) Net ionic equation: 2I (aq) + 2H (a The Catalytic Decomposition of Hydrogen Peroxide, II This demonstration is based on the decomposition of hydrogen peroxide into water and oxygen gas. Reactions like these that are both oxidations and reductions are known as disproportionation reactions: 2 H 2 O 2 (aq) -> 2 H 2 O (l) + O 2 (g) Catalytic Decomposition of Hydrogen Peroxide by Potassium ... The decomposition of hydrogen peroxide in the presence of iodide ion occurs in two steps: H 2 O 2 (aq) + I- (aq) = H 2 O (l) + OI- (aq) H 2 O 2 (aq) + OI- (aq) = H 2 O (l) + O 2 (g) + I- (aq) Materials Preparation: 20 mL 30% hydrogen peroxide, available from chemical supply establishments; Potassium iodide 2 M - Prepare stock solution by ... [Solved] Please see attachments for details | Course Hero The gas phase decomposition of hydrogen peroxide at 400 °C "202(9)—'H20(Q) + 1/2 02(9) is second order in H202 with a rate constant of 0.650 M"1 5—1. If the initial concentration of H202 is 0.236 M, the concentration of H202 will be S M after 20.4 seconds have passed. ...
Decomposition of Hydrogen Peroxide Lab Answers ... The decomposition of hydrogen peroxide by itself is. 2H 2 O 2(aq)-> 2H 2 O (l) + O 2(g). However, this was not the exact reaction that took place. We added KI to the hydrogen peroxide because KI is a known catalyst and it would speed up the reaction. Catalysts are defined by being substances that increase (or decrease) the rate of a chemical ... PDF The Decomposition of Hydrogen Peroxide By A. The velocity of decomposition of hydrogen peroxide is propor- tional to the concentration of catalase. B. In dilute solutions of peroxide (up to 0.1 N) the velocity of reac- tion is proportional to the concentration of hydrogen peroxide, but as the peroxide concentration is increased the velocity becomes independ- Decomposition of hydrogen peroxide equation What is the rate law for decomposition of hydrogen peroxide? The decomposition of hydrogen peroxide is a 1st order reaction with k = 7.30 x 10-4 s-1. What are 2 examples of decomposition reactions? Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen, and the breakdown of water to hydrogen and oxygen. What Is a Decomposition Reaction? - ThoughtCo 12.01.2019 · 2 HAnother example of this type of reaction is the spontaneous decomposition of hydrogen peroxide into water and oxygen: 2 HThe decomposition of potassium chlorate into potassium chloride and oxygen is yet another example: 2 KClO Featured Video. Cite this Article Format. mla apa chicago. Your Citation. Helmenstine, Anne Marie, Ph.D. "Decomposition …
› b-decomposition-reactions5 examples of decomposition reaction - Entrancei Since hydrogen peroxide decomposes in the presence of light, that is why, hydrogen peroxide is kept in coloured bottles so as to cut off light. Decomposition reactions are called the opposite of combination reactions
PDF Decomposition Reactions - Winston-Salem/Forsyth County Schools A + B In this equation, AB represents the reactant that begins the reaction, and A and B represent the products of the reaction. The arrow shows the direction in which the reaction occurs. Q: What is the chemical equation for the decomposition of hydrogen peroxide (H 2O 2) to water (H 2O) and oxygen (O 2)?
H2o2 decomposition equation - estebantorreshighschool.com Hydrogen peroxide can easily break down, or decompose, into water and oxygen by breaking up into two very reactive parts - either 2OHs or an H and HO2: If there are no other molecules to react with, the parts will form water and oxygen gas as these are more stable than the original molecule, H2O2. How long does h2o2 take to decompose?
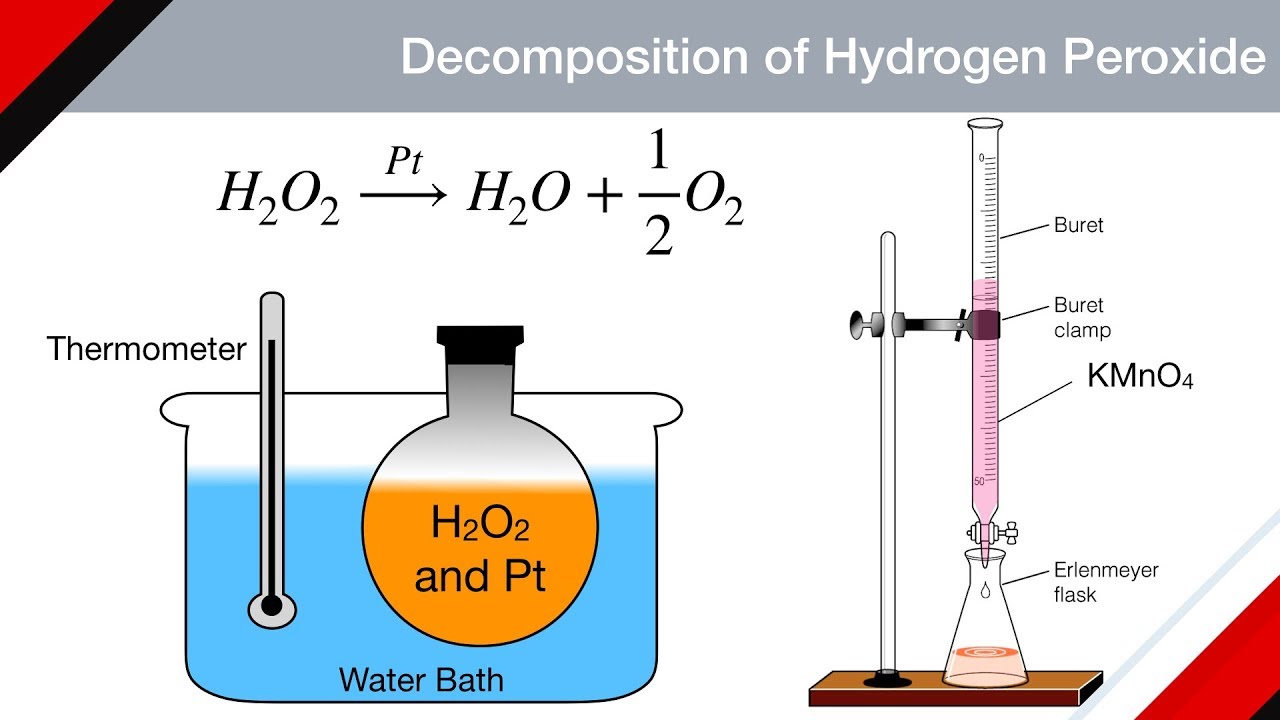
PDF The Decomposition of Hydrogen Peroxide - Chem21Labs Decomposition of Hydrogen Peroxide The decomposition of hydrogen peroxide in aqueous solution proceeds very slowly. A bottle of 3% hydrogen peroxide sitting on a grocery store shelf is stable for a long period of time. The decomposition takes place according to the reaction below. 2 H 2 O 2 (aq) 2 H 2 O O 2 (g)
The role of adsorbed hydroxide in hydrogen evolution ... Oct 19, 2020 · The bifunctional mechanism that involves adsorbed hydroxide in the alkaline hydrogen oxidation and evolution reactions, important in hydrogen fuel cells and water electrolysers, is hotly debated.
How to Balance H2O2 = O2 + H2O: Decomposition of Hydrogen ... In order to balance H2O2 = O2 + H2O you'll need to watch out for two things. First, be sure to count all of H and O atoms on each side of the chemical equat...
UNIT 6 - CHEMICAL REACTIONS - Weebly hydrogen and nitrogen monoxide yields water and nitrogen : 9. sulfur and oxygen yields sulfur trioxide : 10. calcium carbonate yields calcium oxide and carbon dioxide : 5 . Worksheet #3 . More Balancing . Write and balance the following equations. Identify the reaction type. 1. magnesium and hydrogen chloride produce hydrogen and magnesium chloride . 2. calcium hydroxide …
What Is the Balanced Equation of the Decomposition ... The balanced equation of the decomposition reaction of hydrogen peroxide is that 2H2O2 decomposes into the products 2H2O + O2 (g). The resulting products are water and oxygen gas. However, the decomposition takes place very slowly. This is an experiment most students in chemistry lab are familiar with.
chemed.chem.purdue.edu › genchem › topicreviewOxidation-Reduction Equations - Purdue University We can therefore add water molecules or hydroxide ions to either side of the equation, as needed. The following equation describes the reaction between the permanganate ion and hydrogen peroxide in an acidic solution. 2 MnO 4-(aq) + 5 H 2 O 2 (aq) + 6 H + (aq) 2 Mn 2+ (aq) + 5 O 2 (g) + 8 H 2 O(l)
What is the chemical equation for the decomposition of ... This is because the small piece of liver acts as a catalyst, or the cause, of the decomposition of the Hydrogen Peroxide.The balanced equation is thus:2H2O2 (Hydrogen Peroxide) -----> 2H2O + O2
socratic.org › chemistry › stoichiometryMole Ratios - Chemistry | Socratic Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations.. For example, in the reaction . 2H₂(g) + O₂(g) → 2H₂O(g) The mole ratio between O₂ and H₂O is #(1 mol O₂)/(2 mol H₂O)#.
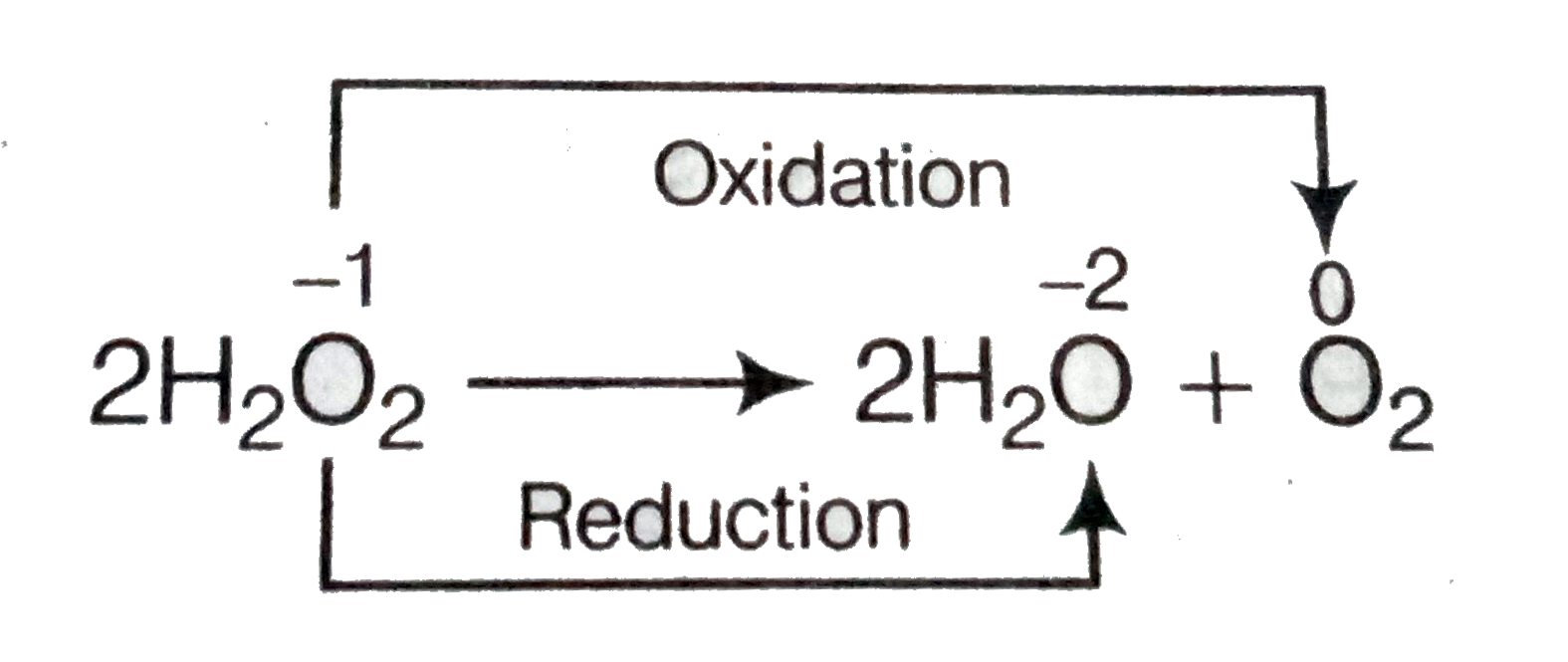
PDF Decomposition of hydrogen peroxide - kinetics and review ... Decomposition of hydrogen peroxide Hydrogen peroxide is a very unique substance due to its molecular structure. It consists atoms of oxygen in oxidation state of -1 unlike many substances, where oxygen occurs in oxidation state of 0 or -2. This means that
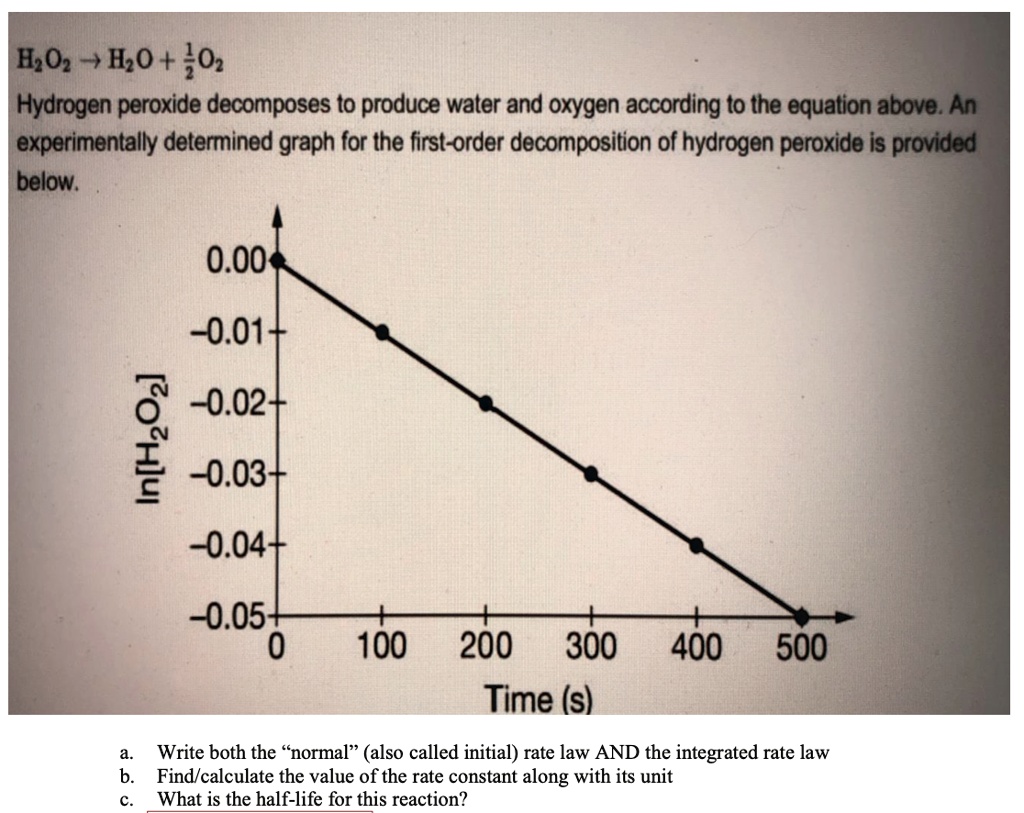
Answered: For the decomposition of hydrogen… | bartleby For the decomposition of hydrogen peroxide in dilute sodium hydroxide at 20 °C 2 H202(aq)-→2 H20(I) + 02(g) the following data have been obtained: [H202], M 8.08x10-2 4.28x10-2 2.27x10-2 1.20x10-2 time, min lo 9.63 19.3 28.9 The average rate of disappearance of H202 over the time period from t = 19.3 min to t = 28.9 min is M min 1.
Hydrogen Peroxide Decomposition Half Equation - Tessshebaylo Electrochemical And Photoelectrochemical Water Oxidation For Hydrogen Peroxide Production Xue 2021 Angewandte Chemie Wiley Library. Half equation hydrogen peroxide you disproportionation definition how to balance h2o2 o2 h2o source scheme ii reaction for the decomposition of effect initial concentration h 2 h2 balanced wikiwand chemical ideas ...
PDF An equation for the decomposition of hydrogen peroxide is ... An equation for the decomposition of hydrogen peroxide is 2H2O2 € 2H2O€€€€+€€€€O2 (a) €€€€The rate of reaction can be determined by collecting the oxygen formed and measuring its volume at regular intervals. Draw a diagram to show the apparatus that you would use to collect and measure the volume of the oxygen formed
Is the decomposition of hydrogen peroxide second-order ... Decomposition of Hydrogen Peroxide The decomposition of hydrogen peroxide in aqueous solution proceeds very slowly. A bottle of 3% hydrogen peroxide sitting on a grocery store shelf is stable for a long period of time. The decomposition takes place according to the reaction below. 2 H2O2 (aq) 2 H2O O2 (g)
EXPERIMENT 7 Decomposition Kinetics of Hydrogen | Chegg.com This experiment studies the decomposition kinetics of the iodide-catalyzed decomposition of hydrogen peroxide (H2O2) which is thought to proceed through the following mechanisms: 1st step: H2O2 (aq) + (aq) 10 (aq) + H2O (0) 2nd step: H2O2 (aq) + 10 (aq) + (aq) + H2O (l) + O2 (g) Overall reaction equation: 2 H2O2 (aq) + O2 (g) + 2 H2O (1) The ...


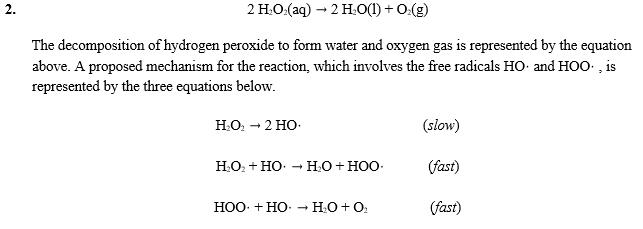








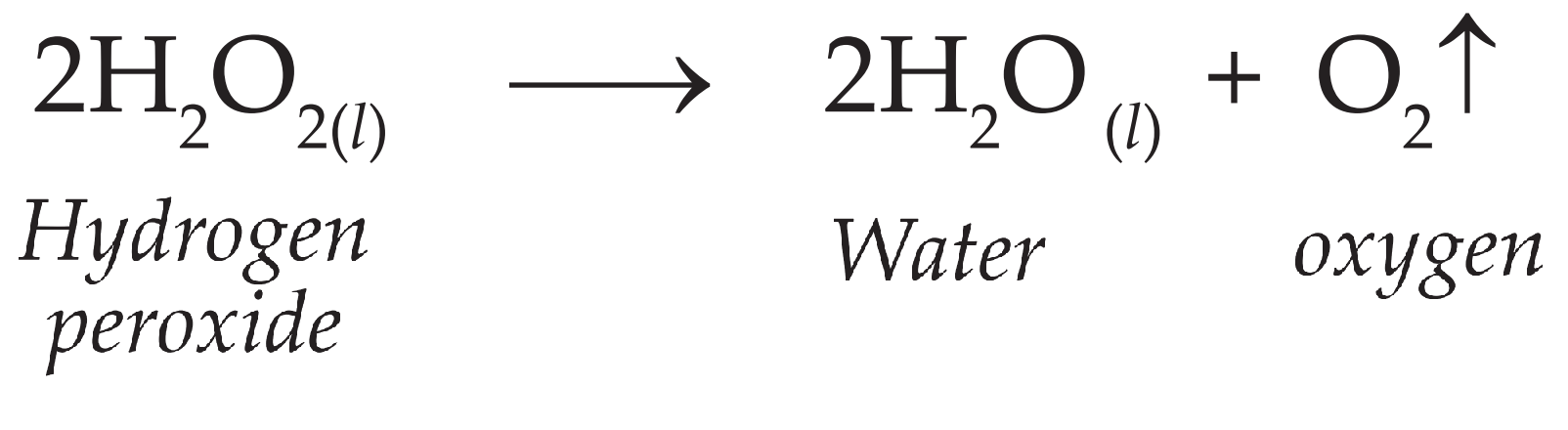





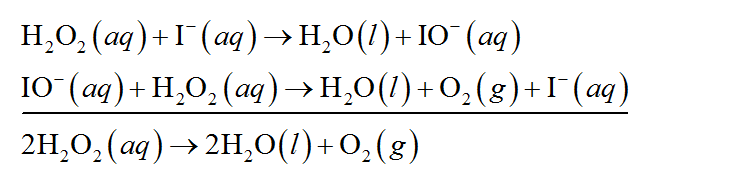
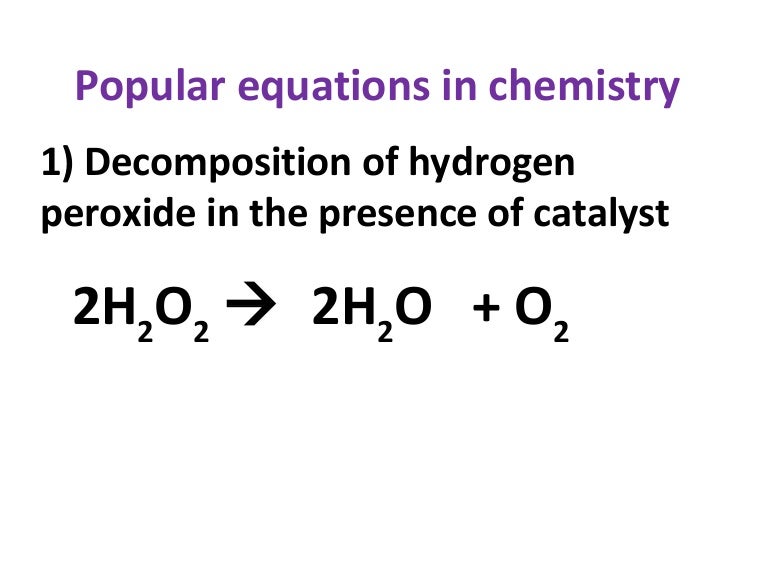








0 Response to "41 decomposition of hydrogen peroxide equation"
Post a Comment